Physical Properties of Alkyl Halides
In the last few articles, we have studied the methods of preparations of alkyl halides. In this article, we shall study the physical properties of alkyl halides. Some physical properties of alkyl halides are as follows:
State:
Lower members (methyl chloride, methyl bromide, methyl fluoride, ethyl bromide, ethyl chloride and ethyl bromide) are gases and higher members are liquids (Up to C18) and solids (Greater than C18).
Odour:
In the pure state, the haloalkanes up to C18 possess pleasant sweet odour. All higher haloalkanes are odourless.
Colour:
Pure haloalkanes are colourless. However, bromoalkanes and iodoalkanes on storing for long period, when exposed to light develop colour.
Boiling Points:
Haloalkanes have higher boiling points as compared to those compared to corresponding alkanes. This is due to their polarity and strong dipole-dipole attractive interaction between haloalkane molecules and greater magnitude of van der Wall’s forces.
- For the same alkyl group the boiling points of haloalkanes are in the order RCl < RBr < RI, because with the increase in the size of halogen atom the magnitude of van der Wall forces of attraction increases.
- Among isomeric alkyl halides, the boiling point decreases with an increase in branching in the alkyl group, because with branching the molecule attains a spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary
- For the same halogen, the boiling point increases with the increase in the molecular mass because with the increase in the size of the alkyl group the magnitude of van der Wall forces of attraction increases. i.e R – X < R -CH2-X < R -CH2-CH2-X
- As the number of halogen in a molecule increases the boiling point of the compound increases because of the increase in the number of halogen atoms the magnitude of van der Wall forces of attraction increases. i.e. CH3Cl < CH2Cl2< CHCl3< CCl4
Solubility:
Alkyl halides are polar in nature (dipole moment 2.05 to 2.15 D) but they are not able to form hydrogen bonds with water molecules. Hence they are sparingly soluble in water. But they are soluble in organic solvents like alcohols, ethers and benzene.
Density:
Alkyl chlorides are generally lighter than water, while alkyl bromides and alkyl iodides are heavier than water. The order of density is RI > RBr > RCl. Poly chlorides are heavier than water. Thus the density of alkyl halides increases with the increase in the number and atomic mass of the halogen atoms. Methyl iodide is the heaviest of all the haloalkanes.
Scientific Reasons:
Density:
Arrange the following in the order of decreasing density. 1-Chloropropane, 1-Iodopropane, 1-Bromopropane
- For the same alkyl group, the density of alkyl halides increases with the increase in the number and atomic mass of the halogen atoms. The atomic mass of I > Atomic mass of Br >Atomic mass of Cl.
- Hence boiling point of 1-Iodopropane > 1-Bromopropane > 1-Chloropropane.
Arrange each of the following set of compounds in the order of increasing densities:
- The order of density is RI > RBr > RCl. Poly chlorides are heavier than water. Thus the density of alkyl halides increases with the increase in the number and atomic mass of the halogen atoms.
- Hence, the order of densities is CH3Cl. < CH2Cl2< CHCl3< CCl4.
- The order of density is RI > RBr > RCl. Thus the density of alkyl halides increases with the increase in the number and atomic mass of the halogen atoms.
- Hence, the order of densities is C2H5Cl < C2H5Br < C2H5I.
Which alkyl halide has the highest density and why?
- For the same alkyl group, the order of density is R-I > R-Br > R-Cl. Thus R-I will have the highest density.
- For the same halogen group, with the increase in the branching, the molecule acquires the spherical shape with less surface area. Thus the tertiary butyl group will have the smallest size.
- From the above two points, we can say that tertiary butyl iodide should have the highest density.
Boiling Points:
Which isomer of C5H11Cl has the highest boiling point? Why?
- Consider the following two isomers of C5H11Cl

- Among isomeric alkyl halides, the boiling point decreases with an increase in branching in the alkyl group.
- 1-Chloropentane is a straight-chain isomer. It has the strongest interparticle forces. Hence it has the highest boiling point among all the isomers. While 1-Chloro-2,2-dimethylpropane has the highest number of branches in all possible isomers, hence it has the weakest interparticle forces. Hence it has the lowest boiling point among all the isomers.
Which isomer of C4H9Cl has the highest boiling point? Why?
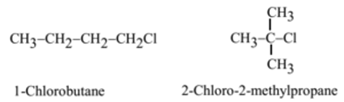
- Among isomeric alkyl halides, the boiling point decreases with an increase in branching in the alkyl group.
- 1-Chlorobutane (n-Butyl chloride) is a straight-chain isomer. It has the strongest interparticle forces. Hence it has the highest boiling point among all the isomers. While 2-Chloro-2-methylpropane (tert-Butyl chloride) has the highest number of branches in all possible isomers, hence it has the weakest interparticle forces. Hence it has the lowest boiling point among all the isomers.
Arrange in the order of increasing boiling points.
Bromobenzene. chlorobenzene, iodobenzene:
- The boiling points of mono halogen derivatives of benzene follow the order Iodo > Bromo > Chloro.
- Hence boiling point of Chlorobenzene < Bromobenzene < Iodobenzene .
n-pentyl chloride, iso-pentyl chloride, neo-pentyl chloride:
- Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary
- Hence boiling point of neo-pentyl chloride < iso-pentyl chloride < n-pentyl chloride.
Bromomethane, Bromoform, Chloromethane, Dibromomethane:
1-Chloropropane, isopropyl chloride, 1-Chlorobutane:
- As the number of halogen in a molecule increases the boiling point of the compound increases. Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains a spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary.
- Hence boiling point of isopropyl chloride < 1-Chloropropane < 1-Chlorobutane.
Methyl chloride, methyl bromide, methyl iodide:
Methyl bromide, methylene bromide, bromoform:
- As the number of halogen in a molecule increases the boiling point of the compound increases.
- Hence the order of boiling points is methyl bromide (CH3Br) < methylene bromide (CH2Br2) < Bromoform (CHBr3).
Propane, n-propyl bromide, isopropyl bromide:
- Haloalkanes have higher boiling points as compared to those compared to corresponding alkanes. This is due to their polarity and strong dipole-dipole attractive interaction between haloalkane molecules.
- Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains a spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary.
- Hence the order of boiling points is propane (alkane) < isopropyl bromide (isoalkyl halide) < n-propyl bromide (primary alkyl halide).
n-butyl chloride, iso-butyl chloride, tert-butyl chloride:
- Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains a spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary.
- Hence the order of boiling points is tert-Butyl chloride < iso-butyl chloride < n-butyl chloride.
1-Bromopropane, isopropyl bromide, 1- Bromobutane:
- Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases. The order of boiling point is Primary > Secondary >= iso > Tertiary. or the same halogen, the boiling point increases with the increase in the molecular mass.
- Hence the order of boiling points is isopropyl bromide < 1-Bromopropane < 1- Bromobutane
Explain why 1-Chlorobutane has higher B.P. than 2-Chlorobutane?
Among isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl group, because with branching the molecule attains spherical shape with less surface area. As a result, interparticle forces become weaker. Hence the boiling point decreases.
The order of boiling point is Primary > Secondary >= iso > Tertiary. Hence 1-Chlorobutane (primary alkyl halide) has higher B.P. than 2-Chlorobutane (secondary alkyl halide).
Explain why Bromoethane has a higher boiling point than Chloroethane. OR Out of ethyl bromide and ethyl chloride which has a higher boiling point and why?
Solubility:
Explain why choroform is not soluble in water although it is polar. OR alkyl halides though polar, are immiscible with water.
- A substance is soluble in water if its molecules are capable of forming hydrogen bonds with water. Chloroform molecules do not form the hydrogen bond with water.
- The energy required to break the bonds between haloalkane molecules is much larger than the energy released during the formation of the bond between haloalkane molecules and water molecules. Hence chloroform is not soluble in water although it is polar.
Alkyl halides are insoluble in water though they contain polar C-X bond. Explain.
- Alkyl halides are polar in nature but they are not able to form hydrogen bonds with water molecules. Hence they are sparingly soluble in water. But they are soluble in organic solvents like alcohols, ethers and benzene.
Dipole Moment:
Which one of the following has the highest dipole moment?
- The dipole moment of CH2Cl2 is the highest while that of CCl4 is zero. Dipole moment of CH2Cl2 is greater than CHCl2 because the dipole moment of the third C-Cl bond of CHCl3 opposes the dipole moment of the remaining two C-Cl bonds.
Science > Chemistry > Organic Chemistry > Halogen Derivatives of Alkanes > Physical Properties of Alkyl Halides
- Tags Alkyl halides, Boiling points of alkyl halides, Chemistry, Chemistry of carbon compounds, Density of alkyl halides, Dihalogen derivatives of alkanes, Dipole moment, Halogen derivatives of alkanes, Monohaloen derivatives of alkanes, Organic chemistry, Polyhalogen derivatives of alkanes, Solubility of alkyl halides, Trihalogen derivatives of alkanes



